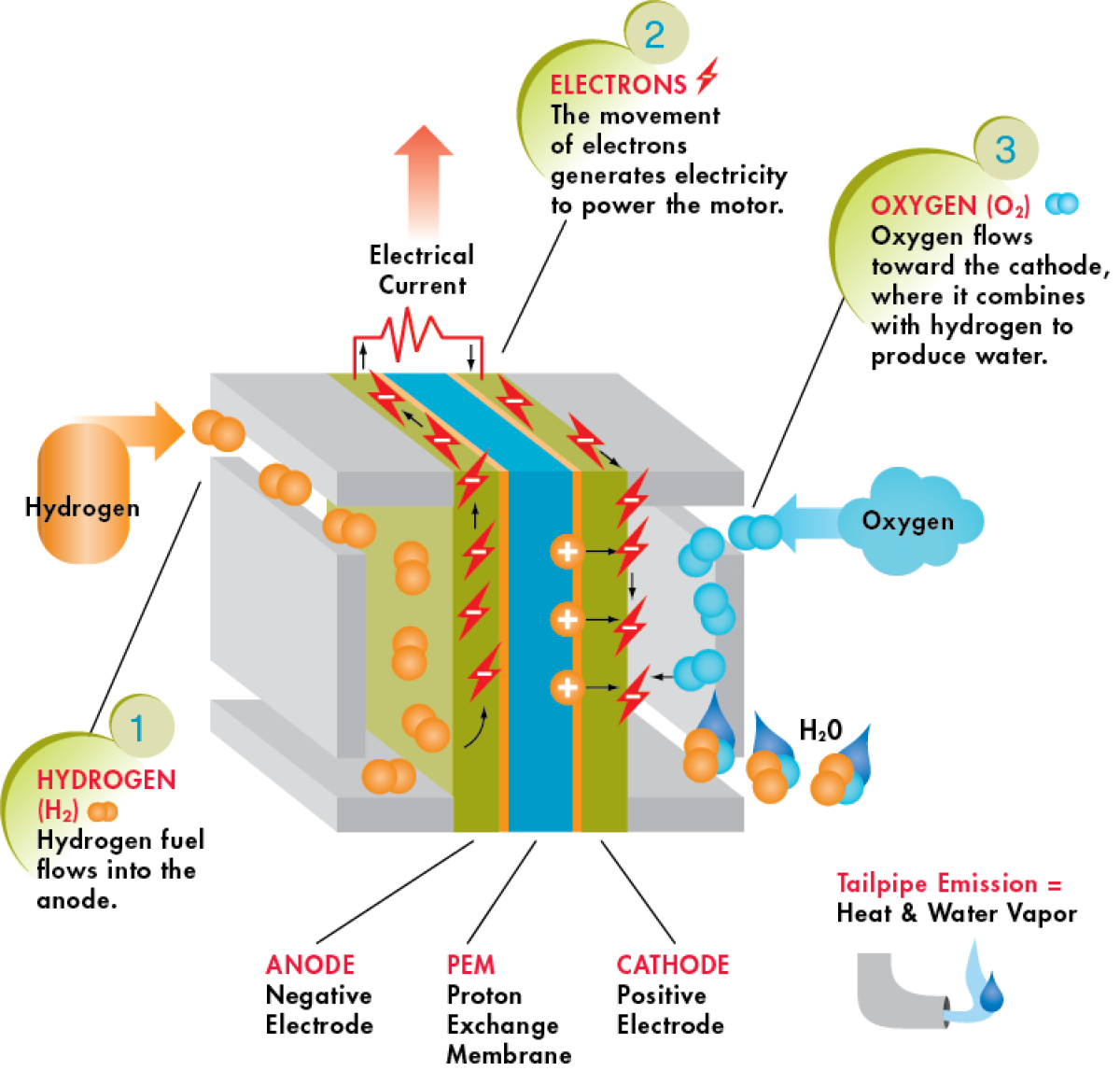
The fuel cell is a electric power generation device. The type of fuel cell used in trucks are a proton exchange membrane (PEM) design, which passively generate electricity through the chemical reaction of hydrogen and oxygen with the assistance of a platinum catalyst. Like batteries, fuel cells contain an anode and a cathode, but the difference is these are separated by the PEM.
The platinum catalyst disassociates the hydrogen atom into its component electron and proton, at the anode. The proton can pass through the PEM, however the electron cannot and therefore runs through the vehicle’s electrical circuit. This is the source of electricity that powers the vehicle.
At the cathode, the platinum catalyst disassociates the oxygen molecule, making it receptive to combining with the hydrogen protons as they pass through the PEM and electrons at the end of the circuit, forming H2O (water). The majority of energy in this reaction is captured as electrical energy and the amount of heat generated is significantly less than in the case of hydrogen combustion.