Storage Options (Compressed Gas vs. Cryogenic Liquid)
As a trucking fuel, the challenge for hydrogen is how to store enough hydrogen onto the truck to achieve needed operational range and carrying capacity without taking away valuable gross vehicle weight. In its natural state, hydrogen is a lighter-than-air gas that must be either highly compressed into gas cylinders or transferred as a cryogenic liquid into dewar-type liquid tanks. All hydrogen storage systems require more space and weight than conventional diesel fuel storage. Currently all commercially available fuel cell tractors are equipped with gaseous hydrogen storage; however significant investment is going into cryogenic liquid hydrogen dispensing and its onboard truck storage.
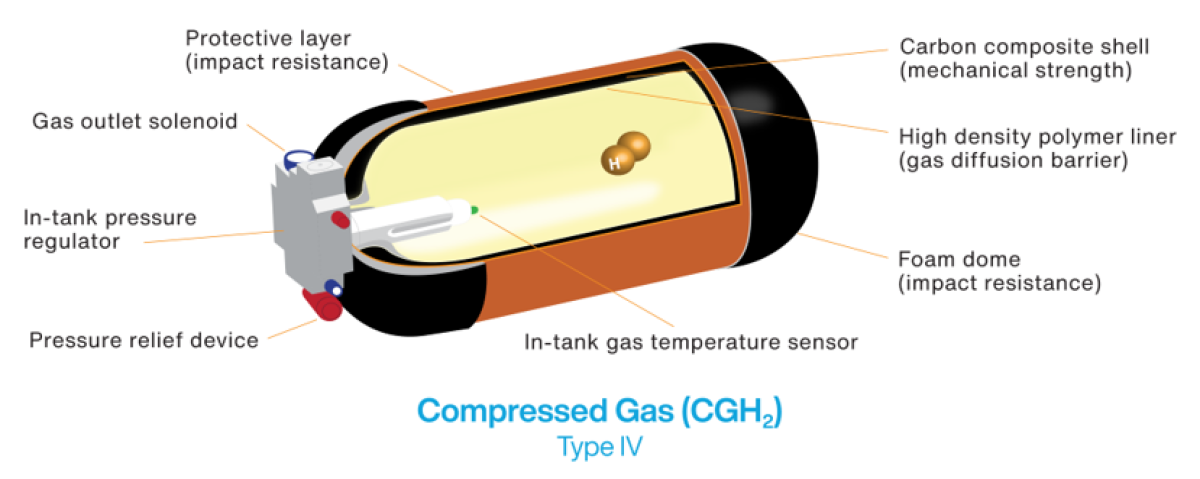
Storage of hydrogen gas requires high-pressure (35 MPa or 70 MPa) composite wrapped tanks to achieve sufficient fuel density. Gaseous hydrogen has the advantage that once pressurized there are fewer mechanical parts on the vehicle. However, gaseous storage also comes with limitations in energy density and storage volume compared to cryogenic storage. Most heavy-duty hydrogen trucks today are designed to accommodate 40-80 kg of gaseous hydrogen storage in an array of four to six gas cylinders; this translates to approximately 300-600 miles.
Hydrogen in liquid form offers the possibility of faster fueling and storage of more mass in a smaller volume as compared withcompressed gas, however with a boiling temperature of -423°F (-253°C), liquid hydrogen requires special handling. In ambient conditions, a phenomenon called boil-off occurs, where liquid hydrogen vaporizes and is potentially lost to the atmosphere. Currently two methods of mitigating liquid hydrogen boil-off are cryo-compression and subcooled liquid hydrogen storage systems.
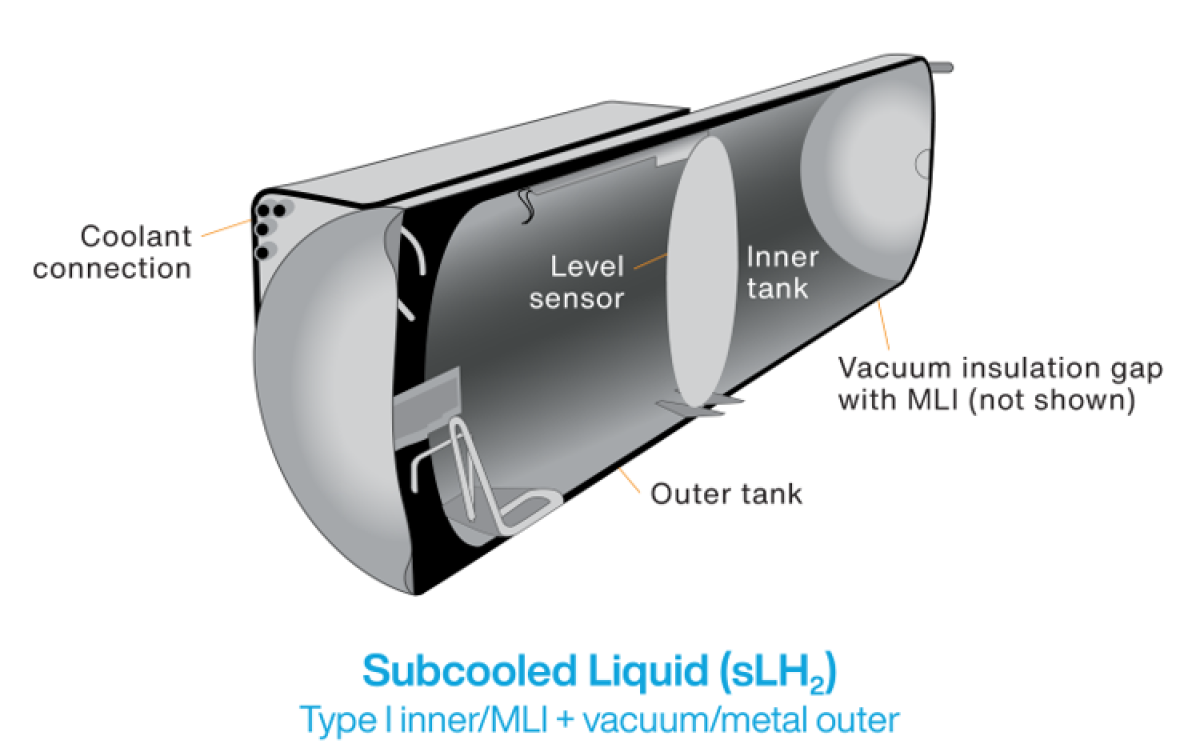
Subcooled liquid hydrogen (sLH2) systems are the newest innovation in this space. These systems slightly increase the pressure of liquid hydrogen (by 1.6 MPa), raising the boiling temperature (albeit more modestly than cryo-compression), stabilizing the cryogenic hydrogen. At this lower pressure the storage tank can be vacuum insulated, double-walled stainless steel construction, without a need for additional carbon fiber reinforcement. The developers of this technology indicate that the lower pressure greatly simplifies the hydrogen refueling process, lowering the total system cost. This is the method demonstrated in 2024 by Daimler Truck and Hyzon (see inset).
Hydrogen physical properties and their impacts on its use as a fuel
- Hydrogen is a gas at room temperature and pressure.
- Hydrogen is the lightest and smallest molecule.
- Hydrogen as a transportation fuel must be stored in a way that increases its energy density, meaning packing more hydrogen fuel into a given space to enable long distance travel. This can be done in two ways, as a highly compressed gas or as a cryogenic liquid.
- Hydrogen transitions from gas to liquid at the cryogenic temperature of -423°F (-253°C) which is called cryogenic liquid hydrogen. Liquid hydrogen does “boil off,” therefore it is best used in high utilization applications.
- Vehicles with gaseous hydrogen fuel on-board: hydrogen is pressurized to either 5,000 psi or 10,000 psi (35 MPa or 70 MPa). The higher pressure allows the use of smaller fuel tanks. Currently, this is the dominant storage technology.
- Hydrogen gas is dispensed expanding the very high pressure gas through the fueling nozzle into a lower pressure holding tank on the vehicle. When hydrogen gas expands, it heats up.
- Vehicles with cryogenic liquid hydrogen on-board: Hydrogen in liquid state has much higher energy density than in its gaseous form and therefore can be stored in smaller volume contains and achieve increased vehicle range. Currently this storage technology is in development.
- Due to the gaseous/liquid forms of dispensing, hydrogen is dispensed by weight (kg) rather than volume.
- One kilogram of hydrogen has the energy equivalent of a gallon of diesel fuel.